




CE Certified HA Dermal Filler
,Cross-Linked HA Dermal Filler
,EPTQ HA Dermal Filler



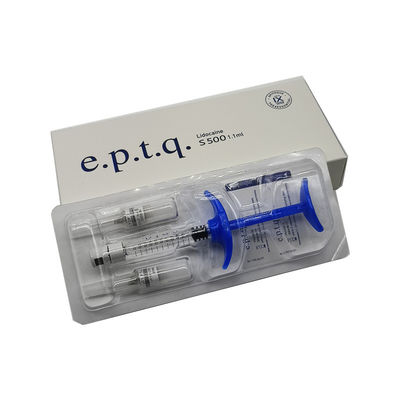
Product description
E.P.T.Q. S100
Indications: Correction of superficial irregularities of the skin profile (fine wrinkles in the forehead and nose bridge, "crow's feet", fine wrinkles around the mouth, periorbital wrinkles). Injection depth: middle layer of dermis (2.5-3 mm).
E.P.T.Q. S300
Medium density filler, based on hyaluronic acid, purified stabilized, 300-conditional viscosity index, without lido .
Indications: Correction of deep irregularities of the skin profile (nasolabial folds, perioral wrinkles, wrinkles on the cheeks, puppet lines; increase in volume and correction of lip contours). Injection depth: medium / deep dermis.
E.P.T.Q. S500
Production: South Korea
Ingredients
Hyaluronate Sodium (Conc.% < 3.0 %), water (Conc.% ≥ 95 %)
Product spection :
| product name | E.P.T.Q filler |
| HA compostion | 24mg |
| volume | 1ml |

THE HYALURONIC ACID FILLER E.P.T.Q. PRODUCED ACCORDING TO TECHNOLOGY AND PROCESS OFJETEMA R&D CENTER PROMISES AN EXQUISITE AND SAFE QUALITY.
The e.p.t.q. filler produced according to“The 9 Process” promises an exquisite product that meets safety standards.
1. Safety of raw ingredients managed in exclusive lines
2. High purity HA content for duration and prevention of swelling
3. High viscoelastic for lift
4. Homogeneous particle implementation technology to complete sophisticated molding
5. No detection of residual chemical catalyst substance through the complete crosslinking process
6. Aseptic manufacturing environment to prevent side effects
7. Less than 0.1 EU/ml of Endotoxin
8. Minimizes pain with the same pH, osmotic pressure as the human body
9. Optimized injection pressure for sophisticated procedures
e.p.t.q. keeps endotoxin below 0.1 EU/ml and no detection of residual BDDE minimizing various side effects that may occur after the procedure.
